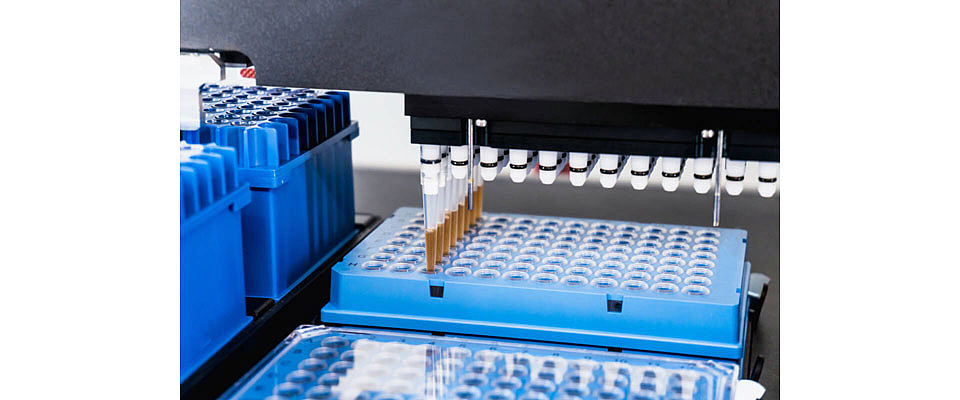
A new SARS-CoV-2 test from Roche makes it possible to quickly analyze a large number of samples for the presence of the novel coronavirus. The plastic consumable materials required for this are produced under cleanroom conditions on machines from KraussMaffei's NETSTAL ELION series.
The novel coronavirus, also known as SARS-CoV-2, currently has the world in its grip. Scientists and pharmaceutical companies are working together under huge pressure and on a global scale in order to make an effective vaccine available as quickly as possible. However, it will take some time before a vaccine providing comprehensive protection is approved and made available to the public. Until then, all countries must concentrate their best efforts on keeping the spread of the coronavirus to an absolute minimum and breaking up any chains of infection. Epidemiologists worldwide have stated that chains of infection may only be effectively broken up if testing is as comprehensive as possible. This is of the utmost importance, because the COVID-19 illness caused by the coronavirus results in mild symptoms or almost none at all in 80% of cases. However, because not enough tests are available right now, most countries are not currently able to test people displaying only mild symptoms. As a result, the true extent of the epidemic is still relatively unknown in most regions, and the actual mortality rate is extremely difficult to calculate.
New Roche quick test improves testing capacities
But there is also good news. The Swiss Roche pharmaceutical group has recently reached a breakthrough in diagnosing the novel coronavirus. A test kit for the fully automatic Cobas 6800 and 8800 diagnosis systems was developed within a very short period of time. Following an expedited process, Roche obtained approval for the new quick test from the US health authority FDA. In Europe, Roche completed a self-certification process on the basis of the data used for the USA.
There are currently around 800 Cobas 6800 and 8800 diagnostic devices installed throughout the globe. These can now be used to test millions of patients per month to determine whether they have caught the virus, or are merely suffering from an ordinary cold or a case of the flu. The incredible performance of the Cobas systems means that testing capacities have been significantly increased. The larger Cobas 8800 can analyze up to 1,056 samples within a 8-hour period. Millions of tests can be made available each month to run on the cobas 6800 and cobas 8800 systems. Roche is now aiming to deliver as many tests as possible and is pushing its production capacity to the limit.